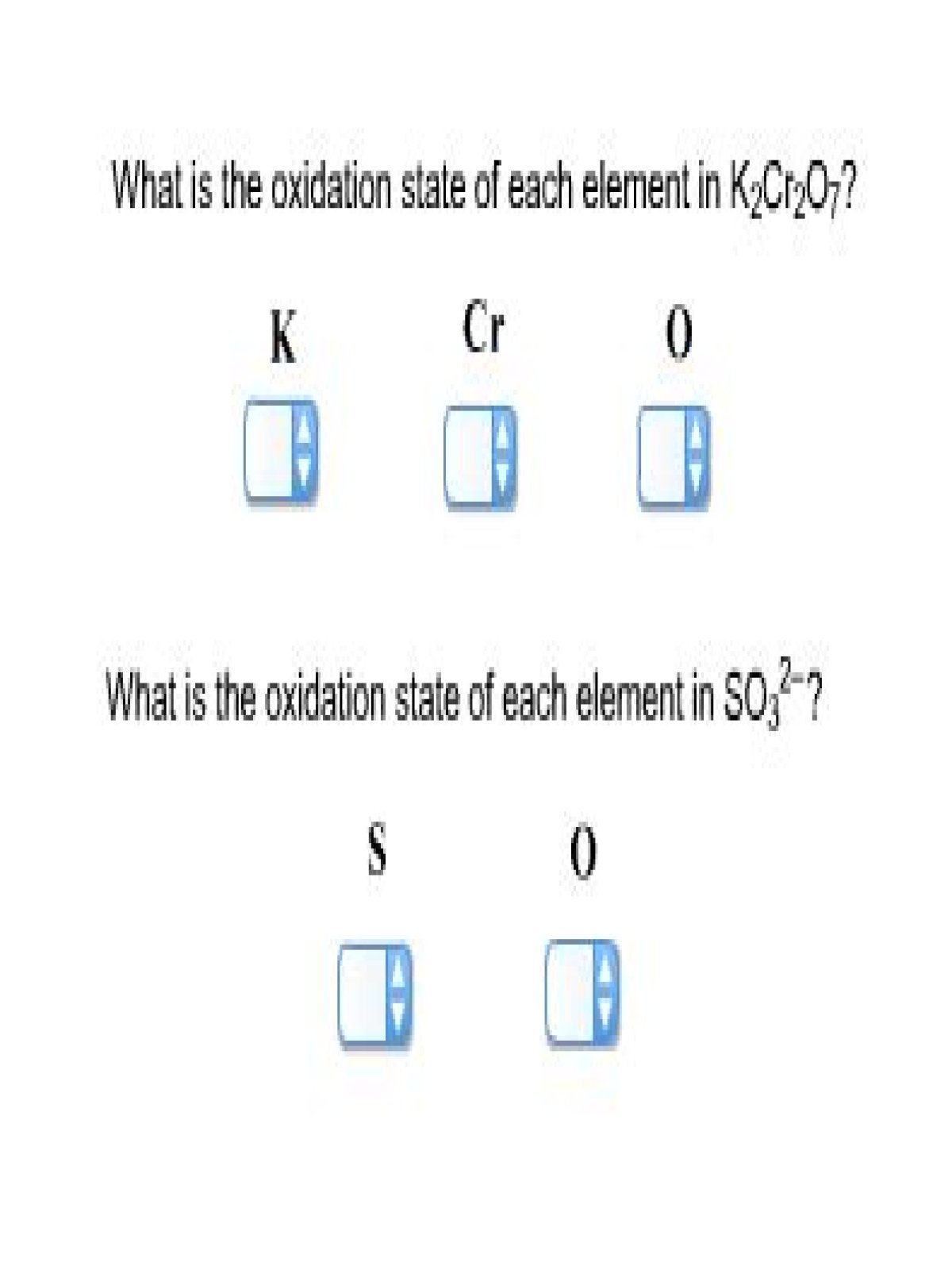
What Is The Oxidation State Of Each Element In K2cr2o7
Did you record the oxidation number of each element in K2Cr2O7? 3
I need to write the oxidation number for each element in K2Cr2O7 and this information is for reference only. What is K2Cr2O7 oxidation and I understand?
K + is only +1 (ionic charge).
The oxidation of each oxygen is number 2.
The oxidation number in Cr is a bit difficult to solve. Isolate ion ions and observe negatively charged decromate 2.
Consider X as the oxidation number in Cr. Suppose there is only one. The combination of oxidation numbers must be in accordance with the ion charge. Define it like this:
x + (7 x 2) = 2
x 14 = 2
x = 12.
Well, if there is only one Cr, then this is the oxidation number. As 2, the corresponding oxidation numbers are 12/2 = +6.
p which helps.
Oxidation number K2cr2o7
K2cr2o7 oxidation index
This page can help you.
D:
Record the oxidation number of each element in K2Cr2O7.
I need to write the oxidation number of each element in K2Cr2O7 and this information is for reference only. What is K2Cr2O7 oxidation and I understand?