Risk-based quality management (RBQM) solutions allow sponsors and CROs to confidently detect, and effectively manage, risk in clinical trials. It is an evidence-backed approach with regulatory backing, meaning wide-scale adoption is becoming an increasing inevitability.
What processes are involved in a risk-based approach to manage quality throughout all stages of the clinical trial?
Step 1: identification of critical processes and data.
What is risk-based management?
Risk-based quality management (RBQM) is a system for managing quality throughout a clinical trial. It will outline how sponsors and contract research organizations (CROs) can harness the power of risk-based trial management, making clinical trials better, faster, and cheaper for the industry and safer for patients.
What is risk management clinical trials?
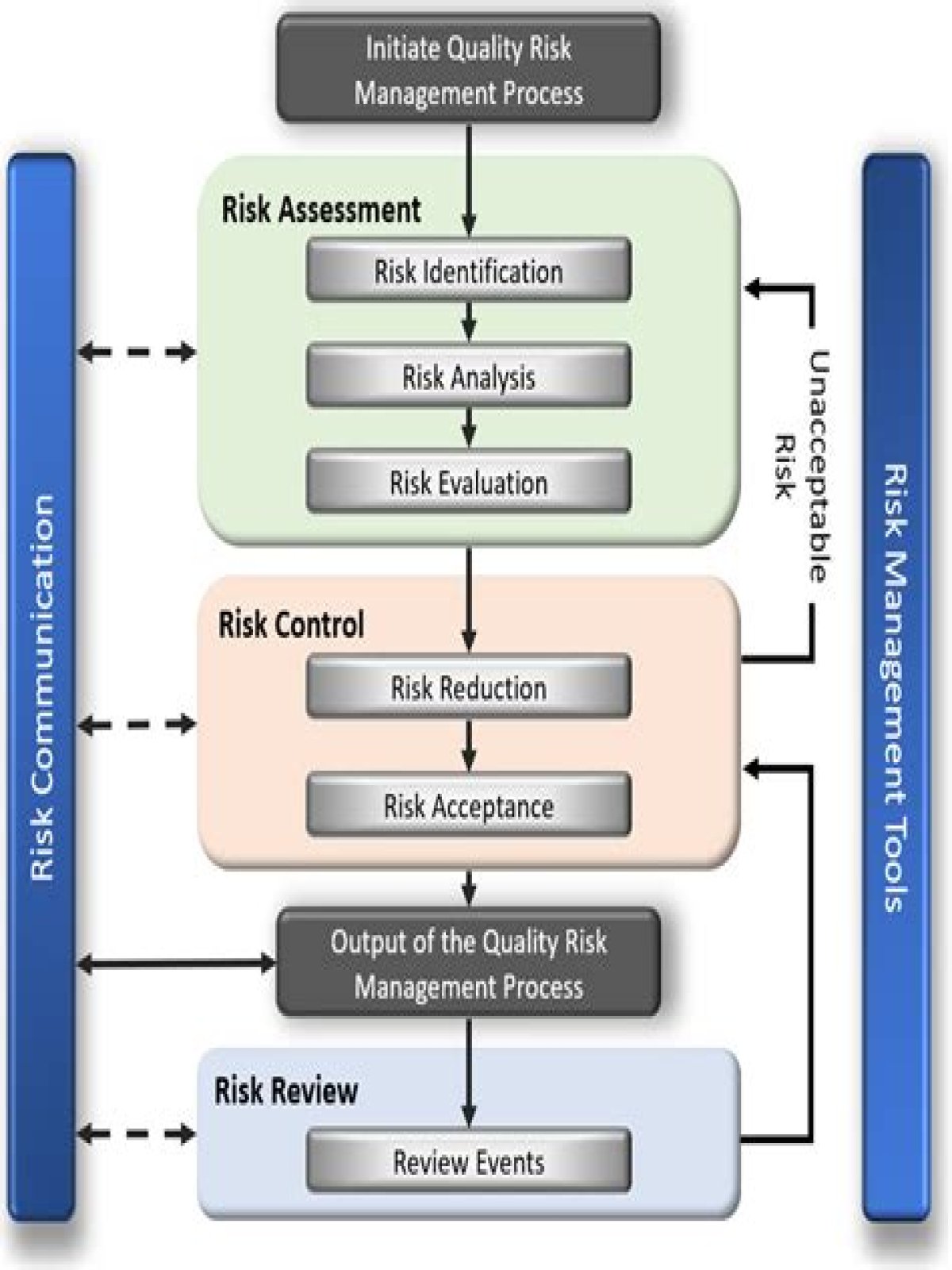
Risk management comprises of a series of activities or processes that are undertaken throughout the life cycle of a clinical trial to identify, evaluate, monitor, control, prevent, mitigate, communicate and review, any factor (or process) that threatens the quality of the trial.
What is a risk based monitoring approach?
Risk-based monitoring is the process of ensuring the quality of clinical trials by identifying, assessing, monitoring and mitigating the risks that could affect the quality or safety of a study.
What is centralized monitoring in clinical trials?
The FDA definition of centralized monitoring states that it: “Consists of a remote evaluation carried out by sponsor personnel or representatives (e.g., clinical monitors, data management personnel, or statisticians) at a location other than the sites at which the clinical investigation is being conducted.”
Why risk management is important in clinical trials?
Enable Proactive Risk Management Risk Management focuses monitoring on trial processes most likely to affect patient safety and data quality so that sponsors, CROs, and investigators can quickly and effectively mitigate risks or address errors before they affect trial quality.
What is ICH and GCP?
ICH-GCP. The ICH-GCP is a harmonised standard that protects the rights, safety and welfare of human subjects, minimises human exposure to investigational products, improves quality of data, speeds up marketing of new drugs and decreases the cost to sponsors and to the public.
What is the purpose of risk based monitoring?
How is risk based monitoring done?
A risk-based monitoring process feeds data from study sites into a dashboard, which then alerts the sponsor to situations that need further investigation. A risk-based monitoring process feeds data from study sites into a dashboard, which then alerts the sponsor to situations that need further investigation.
What is risk-based Quality Management?
Reflection paper on risk based quality management in clinical trials EMA/269011/2013 Page 3/15 A management system used to direct and control an organis ation with regard to quality. It is the system that an organisation uses to manage the quality of their services or products .
What is the EMA reflection paper 2/12?
EMA Reflection Paper for Laboratories that Perform the Analysis or Evaluation of Clinical Trial Samples, 2/12 The European Medicines Agency (EMA) published this guidance. It was adopted by the Good Clinical Practices (GSP) Inspectors Working group on February 28, 2012.
What is quality risk management in clinical trials?
Quality risk management is a systematic process for the assessment, control, communication and review of risks associated with the planning and conduct of clinical trials and clinical development programmes. 10. Quality management system Reflection paper on risk based quality management in clinical trials EMA/269011/2013 Page 3/15
Where is risk-based Quality Management in the Bible of clinical research?
Most importantly, the risk-based quality management approach will also be incorporated within the “bible of clinical research”, i.e. in an addendum to the ICH Guideline for Good Clinical Practice (ICH-GCP). The draft version of the revised ICH-GCP Guideline 6 has already completed the public consultation process.