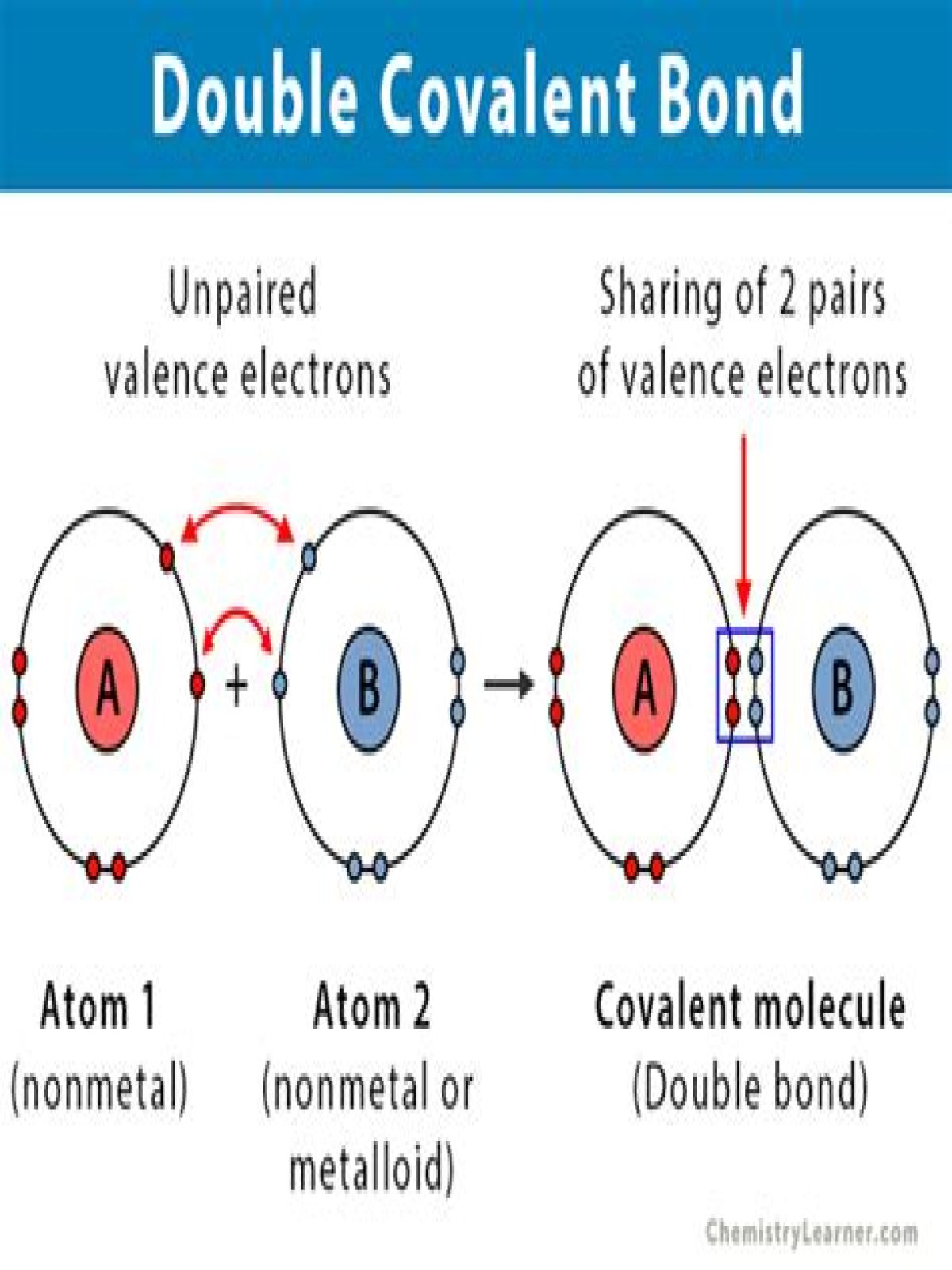
A Double bond is when two atoms share two pairs of electrons with each other.
- How many electrons are shared in each bond single covalent?
- How many electrons are they sharing?
- What is a double covalent bond?
- How many pairs of electrons are shared in a single bond How about in a double bond or triple bond?
- How many electrons are shared in a triple covalent bond?
- What are shared electron pairs?
- How do you find double bonds?
- Which has a double covalent bond?
- What is double bond and triple bond?
- Why is a double bond shorter than a single bond?
- Does a double bond count as two bonding pairs?
- How many electrons do two atoms in a double covalent bond share how many in a triple covalent bond?
- How do you find electron pairs?
- How many electrons are shared in a quadruple covalent bond?
- How are double covalent bond formed?
- Is methane a double covalent bond?
- What is double bond equivalent with example?
- What is alkenes general formula?
- Is double bond and unsaturation equivalent the same?
- How many electrons single electrons not pairs are shared in a double covalent bond?
- How many electrons are in a single double and triple bond respectively?
- What is a double bond quizlet?
- Why do atoms share electrons in covalent bonds quizlet?
- Why is triple bond shorter than double?
- What is the length of a double bond?
- Why is triple bond the shortest?
- Does double bonds count as one bond?
- How many double bonds does so2 have?
- How many double bonds are in a molecule of so2?
Single bonds occur when two electrons are shared and are composed of one sigma bond between the two atoms. Double bonds occur when four electrons are shared between the two atoms and consist of one sigma bond and one pi bond.
How many electrons are they sharing?
In one single bond two electrons are shared. ( one from each atom.) While in a double bond four electrons are shared( two electrons from each atom).
What is a double covalent bond?
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. … Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two.In single bond, 2 electrons are shared, in double bond four electrons are shared and in triple bond six electrons are shared.
triple bond, in chemistry, a covalent linkage in which two atoms share three pairs of electrons, as in the nitrogen molecule, N2, or acetylene, C2H2.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
How do you find double bonds?
DBE = UN = PBoR = C – (H/2) + (N/2) +1, where: C = number of carbon atoms, H = number of hydrogen and halogen atoms, and N = number of nitrogen atoms. One DBE = one ring or one double bond.Which has a double covalent bond?
A molecule with a double covalent bond is oxygen. The oxygen molecule consists of two oxygen (O) atoms. Each oxygen has only six electrons and requires two more to complete its outermost shell. Therefore, the two electrons from each oxygen bond together.
How many electrons are being shared in a double bond to give each oxygen atom an octet?Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter O’s in the O2 Lewis structure represent the nuclei (centers) of the oxygen atoms.
Article first time published onWhat is double bond and triple bond?
When two pairs of electrons are shared (O=O), the bond is called the double covalent bond. Some atoms form triple bonds with one another by sharing three pairs of electrons. For example, nitrogen form a triple covalent bond, by sharing three pairs of electrons with another.
Why is a double bond shorter than a single bond?
Double bonds are shorter than single bonds because double bonds are stronger and therefore pull the electrons closer together in the two elements which decreases the length of the bond.
Does a double bond count as two bonding pairs?
Each double bond uses 2 bond pairs and can be thought of as a single unit. There are 2 double bond units and 1 lone pair, which will try to get as far apart as possible – taking up a trigonal planar arrangement.
A double covalent bond would share 4 electrons and a triple covalent bond would share 6.
How do you find electron pairs?
Find the number of lone pairs on the central atom by subtracting the number of valence electrons on bonded atoms (Step 2) from the total number of valence electrons (Step 1).
A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types double bonds and triple bonds.
How are double covalent bond formed?
A double bond is formed when two atoms share two pairs of electrons. The sharing of two electrons is known as a covalent bond. … Double bonds involve the sharing of electrons between one p orbital of the bonding atoms, as well as the sharing of electrons between the sp orbital of each atom.
Is methane a double covalent bond?
The methane, CH4, molecule composition shows single covalent bonds. The four hydrogen atoms share one electron each with the carbon atom in the methane molecule. … Hydrogen atoms have a bond angle of 109 degrees, giving a tetrahedral geometry to the molecule.
What is double bond equivalent with example?
As in simple words, the double bond equivalent is the number of double bonds and number of triple bonds present in organic molecules. For example, in the case of benzene, there are three double bonds and one ring so its double bond equivalent is four.
What is alkenes general formula?
Alkenes are defined as either branched or unbranched hydrocarbons that possess at least one carbon–carbon double bond (CC) and have a general formula of CnH2n [1].
Is double bond and unsaturation equivalent the same?
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of rings and π bonds.
Two electrons are shared in 1 single covalent bond. Two pairs or four electrons are shared in a double covalent bond. Describe ionic bonding. Ionic bonding involves a complete transfer of one or more electrons from one atom to another; this process forms metal cations and nonmetal anions.
How many electrons are in a single double and triple bond respectively?
Covalent bonding occurs when electrons are shared between atoms. Double and triple covalent bonds occur when four or six electrons are shared between two atoms, and they are indicated in Lewis structures by drawing two or three lines connecting one atom to another.
What is a double bond quizlet?
Double bond. a covalent bond produced by sharing two pairs of electrons between two atoms. Ductility.
Why do atoms share electrons in covalent bonds? to attain a stable noble-gas electron configuration.
Why is triple bond shorter than double?
triple bonds are shorter than double bonds based on the distance between the two atoms sharing 6 electrons between each other.
What is the length of a double bond?
Carbon-Carbon double bond length is ~ 1.34 Å (single bonds in alkane are ~ 1.54 Å. In order to interconvert between the isomers with the methyl groups on the same, and opposite sides, the double bond must be broken. As a result the two isomers do not interconvert at ordinary temperatures.
Why is triple bond the shortest?
The triple bonds are the shortest ones when compared to the double and single bonds. The reason is that the triple bonds are the strongest bonds. (i.e.) The length of the bond is inversely proportional to the bond strength.
Does double bonds count as one bond?
carbon (CH2)—four bonds, no lone pairs; tetrahedral. carbon (CO2)—three bonds (double bond counts as one bond), no lone pairs; trigonal planar. oxygen (OH)—two bonds, two lone pairs; bent (109°)
How many double bonds does so2 have?
There are two double bonds between sulfur atom and oxygen atoms in SO molecule. Also, a lone pair exists on sulfur atom and each oxygen atom has two lone pairs in SO2 lewis structure.
How many double bonds are in a molecule of so2?
Sulfur dioxide has a total of 18 valence electrons: 6 from the sulfur atom and 6 from each of the two oxygen atoms. One way of drawing the molecule’s Lewis structure has the sulfur atom bonded to the two oxygen atoms vi double bonds, with two lone pairs of electrons present on each of the oxygens.