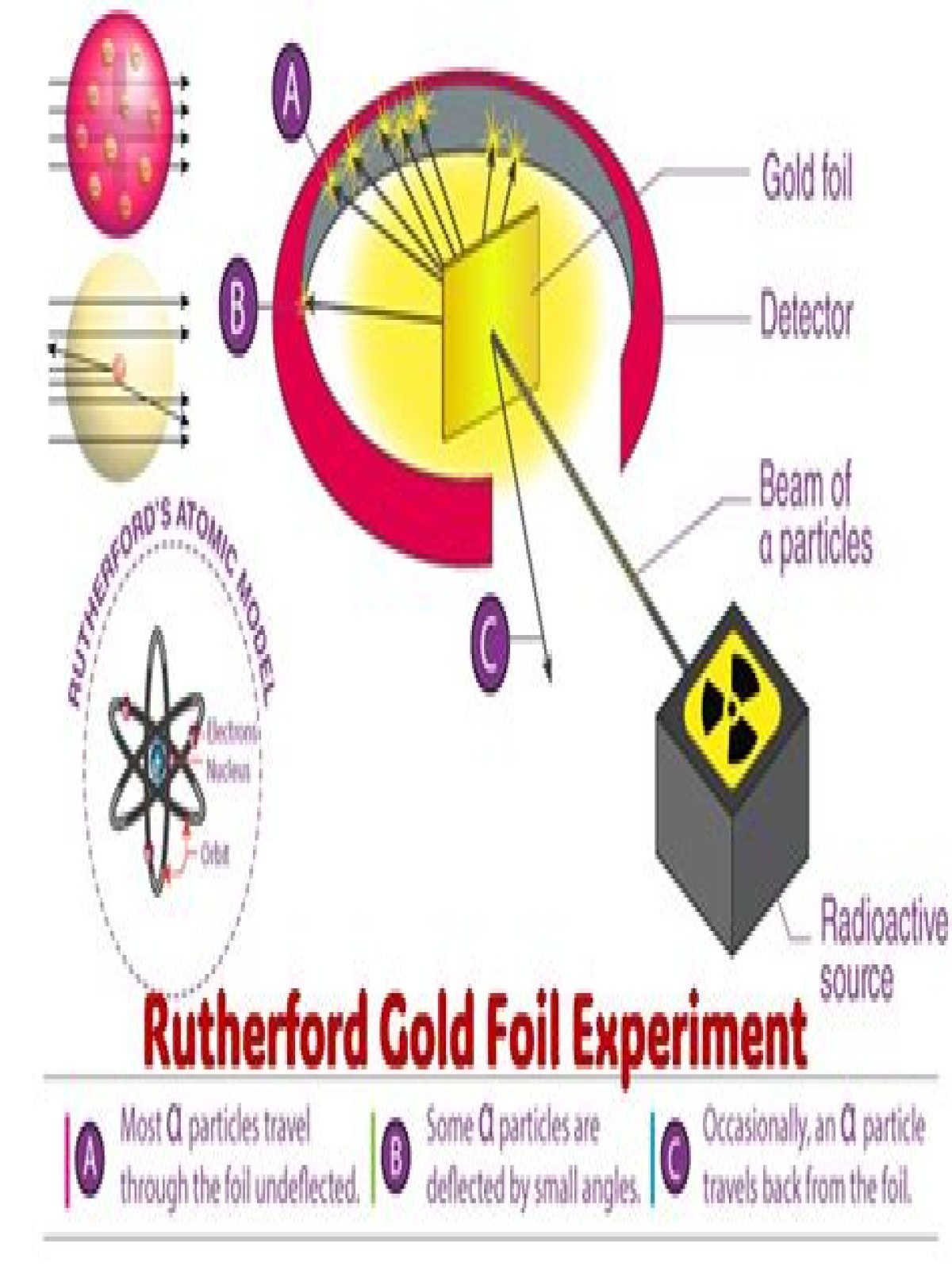
The gold-foil experiment showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Niels Bohr built upon Rutherford’s model to make his own.
- How did Rutherford contribute to the atomic theory?
- How did the results of Rutherford's gold foil experiment differ from his expectations?
- What was the significance of Rutherford's gold foil experiment?
- How did the actual results of the gold foil experiment differ from the expected results?
- When did Ernest Rutherford make his contribution to the atomic theory?
- How did Ernest Rutherford change the atomic model based on his experimental results?
- Why was the gold foil experiment the single most important change in our understanding of atomic structure?
- How did Schrodinger contribute to the atomic theory?
- How did quantum mechanics change our understanding of atoms?
- Why did results surprise Rutherford?
- Which conclusion can be drawn from the results of the experiment showing the scattering of alpha particles by gold foil?
- How did Rutherford change science?
- What caused Rutherford to propose a revised model of the atom How is the Rutherford model different from the previous models?
- What did Schrödinger's equations change about our understanding of electrons?
- What made the scientists change the atomic models?
- Who was Schrödinger and what did he do?
- What were the key conclusions from Rutherford's experiment?
- What occurred during Rutherford's experiment that lead to his discovery of the nucleus?
- What three conclusions came from the gold foil experiment?
- What did quantum mechanics change?
- How has quantum mechanics altered our concept of an atom and its electrons?
- How does quantum mechanics describe an atom?
- Which conclusion is based on the gold foil experiment and the resulting model of the atom?
- What is gold foil experiment name the scientist and the conclusions and shortcomings?
- What conclusion is drawn from Rutherford's scattering experiment of α?
How did Rutherford contribute to the atomic theory?
Ernest Rutherford is known for his pioneering studies of radioactivity and the atom. He discovered that there are two types of radiation, alpha and beta particles, coming from uranium. He found that the atom consists mostly of empty space, with its mass concentrated in a central positively charged nucleus.
How did the results of Rutherford's gold foil experiment differ from his expectations?
How did the results of Rutherford’s gold-foil experiment differ from his expectations? Rutherford expected the alpha particles to pass through the gold foil easily with slight deflection. However, the results showed a majority of the alpha particles passing through completely with no deflection.
What was the significance of Rutherford's gold foil experiment?
Rutherford’s “gold foil experiment” led to the discovery that most of an atom’s mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry.How did the actual results of the gold foil experiment differ from the expected results?
How did the actual results of the gold foil experiment differ from the expected results? … b)There was no difference between the expected and actual results. c)Rutherford expected particles to travel through the atoms, but instead, they ricocheted and rebounded in unexpected directions.
When did Ernest Rutherford make his contribution to the atomic theory?
In 1911, he was the first to discover that atoms have a small charged nucleus surrounded by largely empty space, and are circled by tiny electrons, which became known as the Rutherford model (or planetary model) of the atom.
How did Ernest Rutherford change the atomic model based on his experimental results?
Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
Why was the gold foil experiment the single most important change in our understanding of atomic structure?
Rutherford’s experiment showed that atoms consisted of a dense mass which was surrounded by mostly empty space – the nucleus! … The conclusion that could be formed from this result was that atoms had an inner core which contained most of the mass of an atom and was positively charged.How did Schrodinger contribute to the atomic theory?
Assuming that matter (e.g., electrons) could be regarded as both particles and waves, in 1926 Erwin Schrödinger formulated a wave equation that accurately calculated the energy levels of electrons in atoms.
What are 2 findings conclusions of the gold foil experiment?From the location and number of α-particles reaching the screen, Rutherford concluded the following: i) Almost 99% of the α-particles pass through the gold foil without any deflection. So atom must be having a lot of empty space in it. ii) Several α-particles get deflected at angles.
Article first time published onHow did quantum mechanics change our understanding of atoms?
IF we look at the shell model we get a very 2d view , T he quantum mechanics make us realize there is a lot more to realizing the location of an electron. The big change in understanding that happened with Quantum Physics is the idea that the universe is random, rather than clockwork, at its lowest level.
Why did results surprise Rutherford?
Rutherford discovered the proton, and he also discovered that the atom is mainly empty space. He noticed that a beam of alpha particles was scattered back where it came from by gold atoms, and, since it was known that alpha particles were positive, it was deduced that there was a dense positive core in the nucleus.
Which conclusion can be drawn from the results of the experiment showing the scattering of alpha particles by gold foil?
Conclusion of Rutherford’s scattering experiment: Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected. Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
How did Rutherford change science?
A consummate experimentalist, Rutherford (1871–1937) was responsible for a remarkable series of discoveries in the fields of radioactivity and nuclear physics. He discovered alpha and beta rays, set forth the laws of radioactive decay, and identified alpha particles as helium nuclei.
What caused Rutherford to propose a revised model of the atom How is the Rutherford model different from the previous models?
Rutherford proposed a revised model of the atom because he could not explain the results of his gold foil experiment. He concluded the atom must not be a hollow ball and must have a solid core since the particles (alpha) were deflected at angles while some passed straight through.
What did Schrödinger's equations change about our understanding of electrons?
The Schrödinger wave equation replaced the Bohr ideas about electron location with an uncertainty factor. The location of the electron can only be given as a probability that the electron is somewhere in a certain area.
What made the scientists change the atomic models?
This atomic model has changed over time. Scientists used the model to make predictions. Sometimes the results of their experiments were a surprise and they did not fit with the existing model. Scientists changed the model so that it could explain the new evidence.
Who was Schrödinger and what did he do?
Austrian physicist Erwin Schrödinger was a noted theoretical physicist and scholar who came up with a groundbreaking wave equation for electron movements. He was awarded the 1933 Nobel Prize in Physics, along with British physicist P.A.M. Dirac, and later became a director at Ireland’s Institute for Advanced Studies.
What were the key conclusions from Rutherford's experiment?
What happenedRutherford’s conclusionsA small number of alpha particles were deflected by large angles (> 4°) as they passed through the foil.There is a concentration of positive charge in the atom. Like charges repel, so the positive alpha particles were being repelled by positive charges.
What occurred during Rutherford's experiment that lead to his discovery of the nucleus?
During the experiment, alpha particles bombarded a thin piece of gold foil. The alpha particles were expected to pass easily through the gold foil. … The discovery of the nucleus was a result of Rutherford’s observation that a small percentage of the positively charged particles bombarding the metal’s surface…
What three conclusions came from the gold foil experiment?
Thus the conclusions made were: Atom has a very small nucleus at the centre. There is large empty space around the nucleus. Entire mass of an atom is concentrated in a very small positively charged region which is called the nucleus. Electrons are distributed in the vacant space around the nucleus.
What did quantum mechanics change?
In the Heisenberg-Schrödinger quantum mechanical model of the atom, each electron acts as a wave, or “cloud”) around the nucleus of an atom, with the ability to measure only the speed or position of an electron to a particular probability. This model replaced the Rutherford-Bohr model.
How has quantum mechanics altered our concept of an atom and its electrons?
Quantum mechanics predicted the Bohr atomic model by showing the orbitals of electrons and how they emit energy on jumping from their excited state to another. … Bohr model was only applicable to Hydrogen atom, and it became complex for any other atoms of a specific element and failed to explain chemical bonds.
How does quantum mechanics describe an atom?
Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. … Electrons have an intrinsic property called spin, and an electron can have one of two possible spin values: spin-up or spin-down. Any two electrons occupying the same orbital must have opposite spins.
Which conclusion is based on the gold foil experiment and the resulting model of the atom?
The gold foil experiment led to the conclusion that each atom in the foil was composed mostly of empty space because most alpha particles directed at the foil 1) An atom is mostly empty space with a dense, positively charged nucleus.
What is gold foil experiment name the scientist and the conclusions and shortcomings?
Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.
What conclusion is drawn from Rutherford's scattering experiment of α?
From the observations of this experiment Rutherford concluded that in order to deflect the positively charged alpha particles there must be a repelling force.