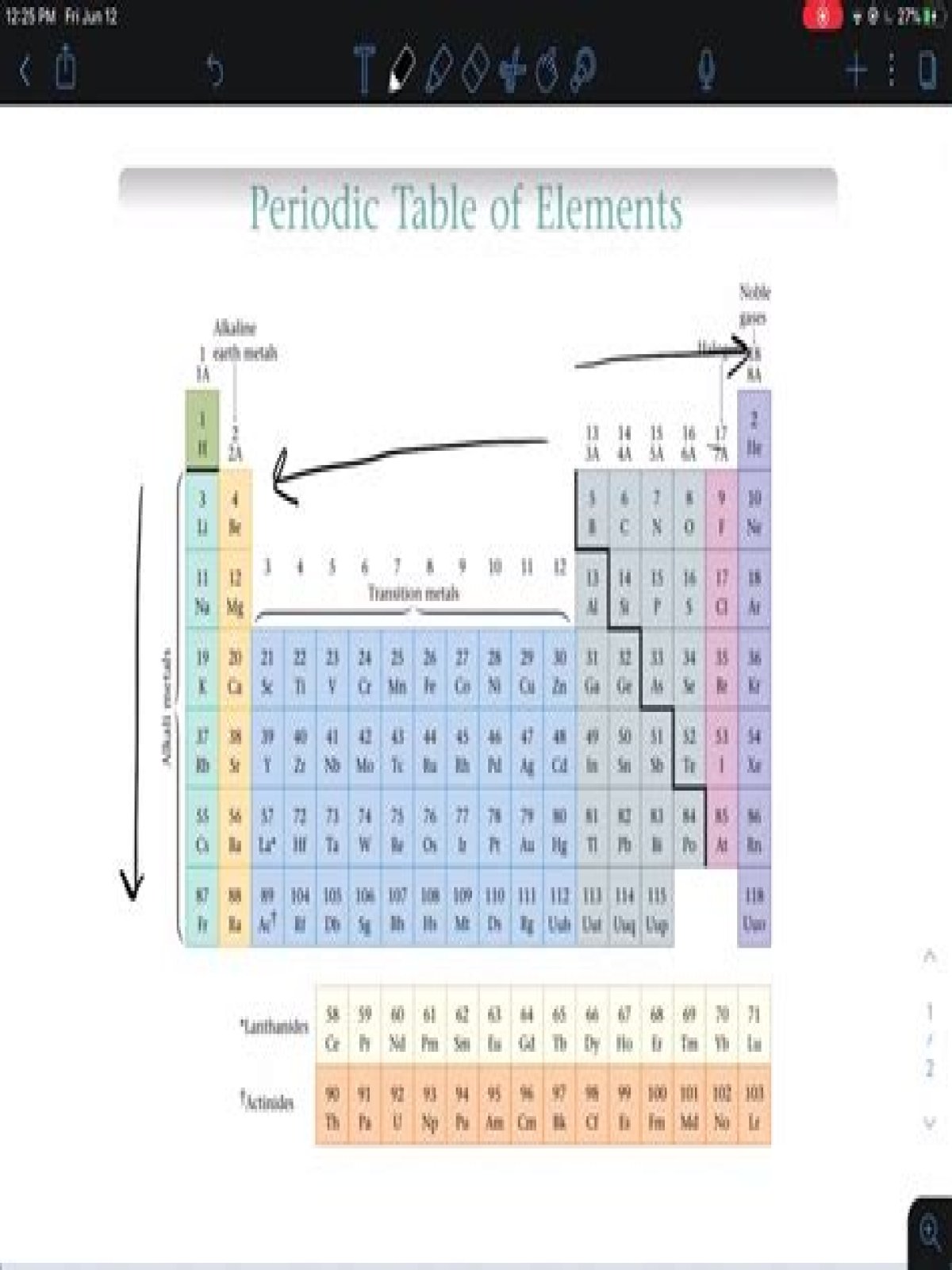
The reactivity of alkali metals increases from the top to the bottom of the group, so lithium (Li) is the least reactive alkali metal and francium (Fr) is the most reactive.
- Why do group 1 elements get less reactive?
- Does group 1 have low reactivity?
- Why Is Lithium the least reactive in group 1?
- Which element of group 1 is most reactive and why?
- Which group of elements is the least reactive?
- Are group 1 metals reactive?
- Which is the least reactive metal?
- Which element in group 1 is least reactive to water?
- What do group 1 elements have in common?
- What is the trend in reactivity in Group 1?
- Why are Group 1 metals called alkali metals?
- Which one is a reactive element?
- Which element is most reactive?
- What groups are most reactive?
- Is group 1 or 2 more reactive?
- How do you know group 1 are metals?
- Why do group 1 elements form +1 ions?
- Which is the least reactive non metal?
- Why is neon the least reactive element?
- Which of the following group of metals are less reactive to the group 1 and 2 of the periodic table?
- How does Group 1 react with water?
- Which is the least reactive metal in the reactivity series?
- Is hydrogen a Group 1 element?
- What are the 2 least reactive metals?
- What is the most and least reactive metal?
- How do you know which element is least reactive?
- What is the only nonmetal in Group 1?
- Which element is least reactive in group 7?
- What is the least reactive element in Group 2?
Why do group 1 elements get less reactive?
the outer electron gets further from the nucleus as you go down the group. the attraction between the nucleus and outer electron gets weaker as you go down the group – so the electron is more easily lost.
Does group 1 have low reactivity?
The reactivity of group 1 elements increases as you go down the group because: the atoms become larger. the outer electron becomes further from the nucleus. … the outer electron is lost more easily.
Why Is Lithium the least reactive in group 1?
Lithium is the least reactive because it is the one with the least electrons. That means the electrons are closer to the nucleus and therefore more attracted to it.Which element of group 1 is most reactive and why?
So, Cesium is the most reactive element in group IA. Note: All the alkali metals react vigorously with halogens to produce salts, as the halogens needs one electron to complete its octet and alkali metals can readily lose electrons to have an oxidation state of +1.
Which group of elements is the least reactive?
Noble gases are nonreactive, nonmetallic elements in group 18 of the periodic table. Noble gases are the least reactive of all elements. That’s because they have eight valence electrons, which fill their outer energy level.
Are group 1 metals reactive?
Alkali metals are among the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of +1.
Which is the least reactive metal?
Gold is the least reactive metal.Which element in group 1 is least reactive to water?
Reactivity of Group 1 Elements The reactivity of alkali metals increases from the top to the bottom of the group, so lithium (Li) is the least reactive alkali metal and francium (Fr) is the most reactive.
Which alkali metal is least reactive?The reactivity of alkali metals increases from the top to the bottom of the group, so lithium (Li) is the least reactive alkali metal and francium (Fr) is the most reactive. Because alkali metals are so reactive, they are found in nature only in combination with other elements.
Article first time published onWhat do group 1 elements have in common?
Group one elements share common characteristics. They are all soft, silver metals. Due to their low ionization energy, these metals have low melting points and are highly reactive. The reactivity of this family increases as you move down the table.
What is the trend in reactivity in Group 1?
The reactivity of Group 1 elements increases as you go down the group because: the atoms get larger as you go down the group. the outer electron gets further from the nucleus as you go down the group.
Why are Group 1 metals called alkali metals?
The alkali metals are so named because when they react with water they form alkalies. Alkalies are hydroxide compounds of these elements, such as sodium hydroxide and potassium hydroxide. Alkalies are very strong bases that are caustic.
Which one is a reactive element?
The most reactive element is fluorine, the first element in the halogen group. The most reactive metal is francium, the last alkali metal (and most expensive element). However, francium is an unstable radioactive element, only found in trace amounts.
Which element is most reactive?
Alkali metals (situated far away from transitional metals and noble gases) are the most reactive elemental group. Cesium is second from the bottom of this group, with 6 electron shells, so it fits all the characteristics of a reactive atom, therefore making it the most reactive element.
What groups are most reactive?
The most reactive metals are the elements in Groups 1 and 2. Elements in Group 1 generally lose an electron so their outer energy level is empty. Elements in Group 2 generally lose two electrons so their outer energy level is empty. These groups easily give up their valence electrons to make a compound.
Is group 1 or 2 more reactive?
The outermost electrons of the alkaline earth metals (group 2) are more difficult to remove than the outer electron of the alkali metals, leading to the group 2 metals being less reactive than those in group 1. These elements easily form compounds in which the metals exhibit an oxidation state of 2+.
How do you know group 1 are metals?
The Group 1 elements are called the alkali metals. They are placed in the vertical column on the left-hand side of the periodic table . All the Group 1 elements are very reactive . … Group 1 elements form alkaline solutions when they react with water, which is why they are called alkali metals.
Why do group 1 elements form +1 ions?
Thus, all the alkali metals have one valence electron, ns1 outside the noble gas core. They readily lose this one electron to attain more stable noble gas configuration and give monovalent M+ ions, where M is any alkali metal. Hence, we can say that alkali metals always form +1 ions.
Which is the least reactive non metal?
Helium is the least reactive non metal. Helium is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert gas.
Why is neon the least reactive element?
Neon, along with helium, argon, krypton and xenon, make up the group known as noble gases. These are the most stable and least reactive elements due to having full valence shells (the outer shell has the max number of electrons, two for helium, eight for the rest).
Which of the following group of metals are less reactive to the group 1 and 2 of the periodic table?
Group 2 consists of the alkaline Earth metals. They are very reactive but less so than the alkali metals. Groups 3–12 contain transition metals. They are less reactive than metals in groups 1 and 2.
How does Group 1 react with water?
All the alkali metals react vigorously with cold water. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. The speed and violence of the reaction increases as you go down the group. This shows that the reactivity of the alkali metals increases as you go down Group 1.
Which is the least reactive metal in the reactivity series?
As we can see in the series, out of all the given options gold is the least reactive metal.
Is hydrogen a Group 1 element?
Alkali metals are the chemical elements found in Group 1 of the periodic table. Although often listed in Group 1 due to its electronic configuration, hydrogen is not technically an alkali metal since it rarely exhibits similar behavior. …
What are the 2 least reactive metals?
Silver, gold, and platinum are metals with the least reactivity.
What is the most and least reactive metal?
ElementReaction with waterLithiumQuicklyCalciumMore slowly
How do you know which element is least reactive?
The reactivity of an element can be determined by looking at the electron configuration of the element. The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium, neon, radon or the transition elements.
What is the only nonmetal in Group 1?
Hydrogen, with its single electron, also lives in Group 1, but the gas is considered a nonmetal.
Which element is least reactive in group 7?
The halogens get less reactive – fluorine, top of the group, is the most reactive element known. Iodine is the least reactive halogen (besides astatine which is often ignored because it is extremely rare).
What is the least reactive element in Group 2?
The least reactive element in group 2 is beryllium.